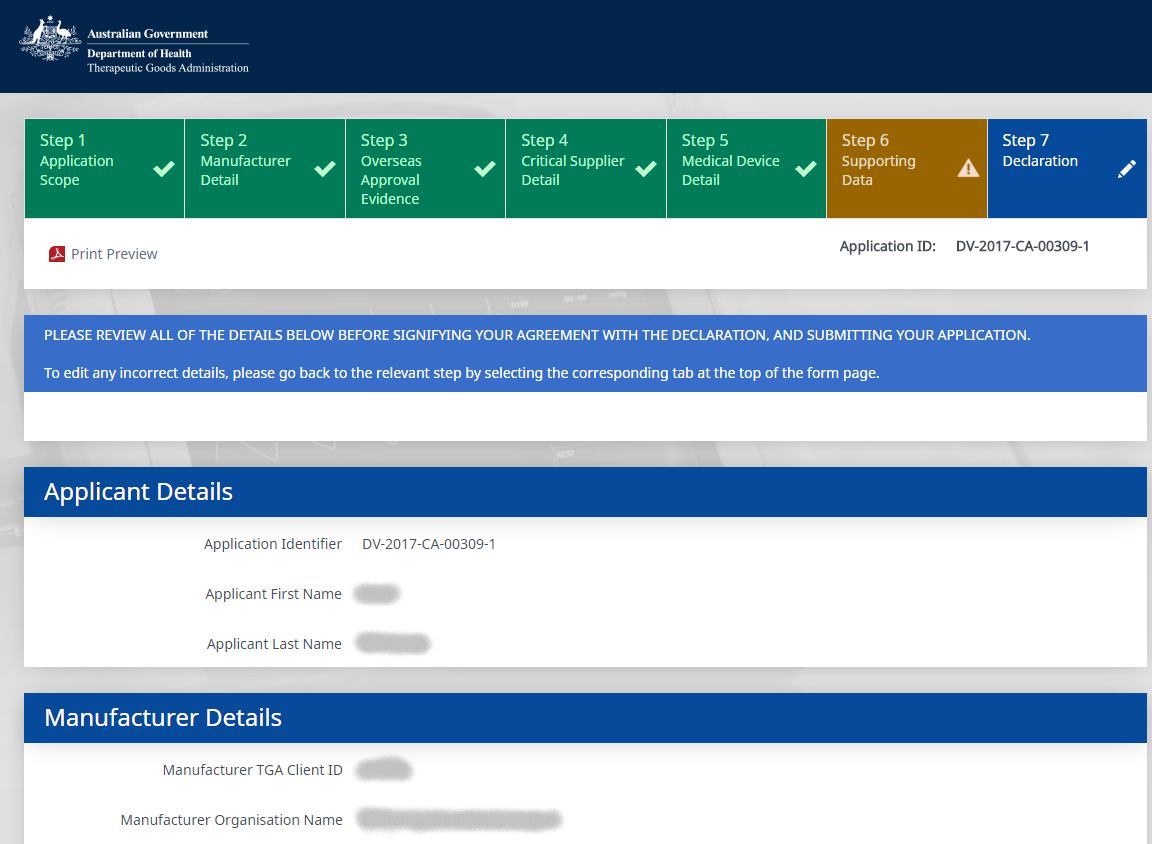
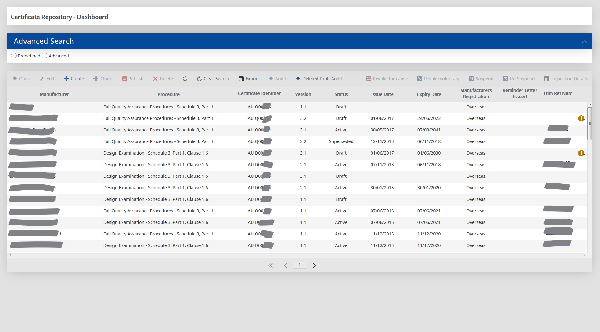
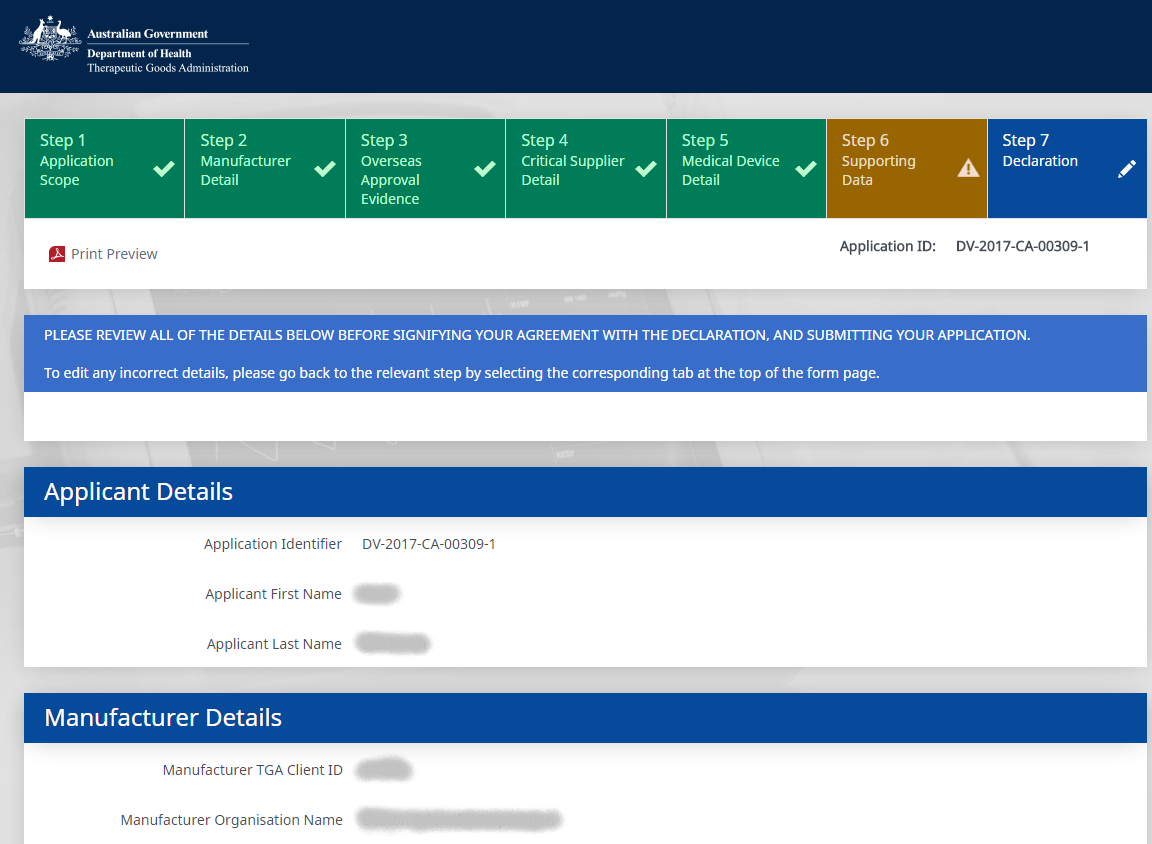
Develop a customer facing, intelligent electronic form to allow clients to submit applications for medical devices Conformity Assessments. Also develop a repository to contain the certificate information and allow the processing of the electronic applications.
Project Details
Client Department of Health
Date June 2017
Skills K2, Forms Develpment, SQL DB develpment. Needs analysis, DevOps
View www,tga,gov,au